Iso and diisocyanates hazards
Taking some special care can help avoid the dangers of iso and diisocyanates
Iso and diisocyanates are the main ingredients in making urethanes as well as some paints, foams, rubbers, and plastics. If your product is made with iso or diisocyanates, you need to understand the dangers of these chemicals, and how to help keep your employees safe.
General Information
Urethanes are made by chemical reactions of iso or diisocyanates. There are several kinds and uses for urethanes. You’ll find urethane foams in packaging, insulation, boat building, furniture, fabrics, and reinforced plastics. Urethane paints, coatings, and films are used in automobile and aircraft manufacturing and provide stable and durable surfaces. Still other urethane formulations are used in electronics.
It’s the mixing of iso and diisocyanates with polyesters and polyethers that make urethane possible. Diisocyanates are chemicals that include the often-used methylene bisphenyl isocyanate (MDI) and toluene 2, 4 diisocyanate (TDI). Lesser known products include hexamethylene diisocyanate (HDI), isophorone diisocyanate (IPDI), and dicyclohexyl methane 4, 4 diisocyanate (HMDI).
This family of chemicals has a high reactivity with polyesters and polyethers, forming urethanes. The reactions happen at room temperature without the addition of other types of energy and cure quickly to form relatively stable urethane products.
But using these chemicals comes with some dangers you need to understand.
Occupational hazards
Diisocyanates are an irritant and can affect your:
Skin
Mucous membrane
Throat
Respiratory tract
Eyes
If you’re exposed, it’s not uncommon to experience a number of symptoms, including:
Burning and watering eyes
Burning in your nose and throat
Cough
Chest tightness
Breathlessness
Temporary reduction in your lung function
Extensive exposure to high concentrations of diisocyanates may also lead to pulmonary edema. While your acute symptoms can fade after getting away from the source of the chemical, permanent damage may still occur.
If you’re in contact with diisocyanates on a regular basis, sensitization is the most significant hazard you face. It’s estimated that 2 to 5 percent of people exposed to diisocyanates will develop some sensitization—including skin and pulmonary sensitization.
If you’ve developed skin sensitization to diisocyanates, you may experience rash, itching, hives, and swelling of the arms and legs. Once sensitized, you don’t have to come into skin contact to have any or all of these reactions. Just being exposed to low concentrations of airborne diisocyanate vapor can cause them. There’s also evidence indicating cross-sensitization, which means you could experience a reaction when exposed to a different diisocyanate.
A pulmonary sensitization will give you a severe allergic reaction similar to asthma, which includes coughing, wheezing, tightness in the chest, and shortness of breath. This type of reaction can sometimes be fatal.
Any sensitization to diisocyanates is irreversible and can’t be cured. Anyone who’s sensitized cannot be in areas where potential exposures to diisocyanates exist.
Recommended controls
Because diisocyanates are highly toxic chemicals, you must reduce or eliminate exposures around your workplace. Here are some ways you can help do that:
Provide personal protective equipment: Require skin and eye protection whenever diisocyanates are present.
Ensure proper exhaust ventilation: Provide a booth or hood with a minimum face velocity of 100 feet per minute (fpm) whenever a urethane process is in use. Provide local exhaust ventilation during packaging operations using urethane foams.
Take precautions when spraying: Perform any spraying of urethane paints, coatings, or foams in a spray booth. Provide spray painters with air-supplied respirators.
Use proper storage containers: Design your storage plan for materials containing raw diisocyanates so it protects containers from heat and direct sunlight.
You’ll also need to take precautions to keep diisocyanates away from bases and caustics, primary or secondary amines, acids or alcohols, and also water. Diisocyanates are very reactive if mixed with any of these and could release significant volumes of carbon dioxide into the air or even result in an explosion. If there’s a fire, you’ll need to explain the dangers to emergency personnel—including the possible release of hydrogen cyanide and carbon monoxide. The best way to put out a diisocyanate-supported fire is with a dry chemical powder, carbon dioxide, or foam. You should only use water if there’s a large supply available.
If diisocyanates spill in your workplace, evacuate the area to avoid exposing any employees. Remember to follow the containment and cleanup recommendations from the Safety Data Sheets (SDS).
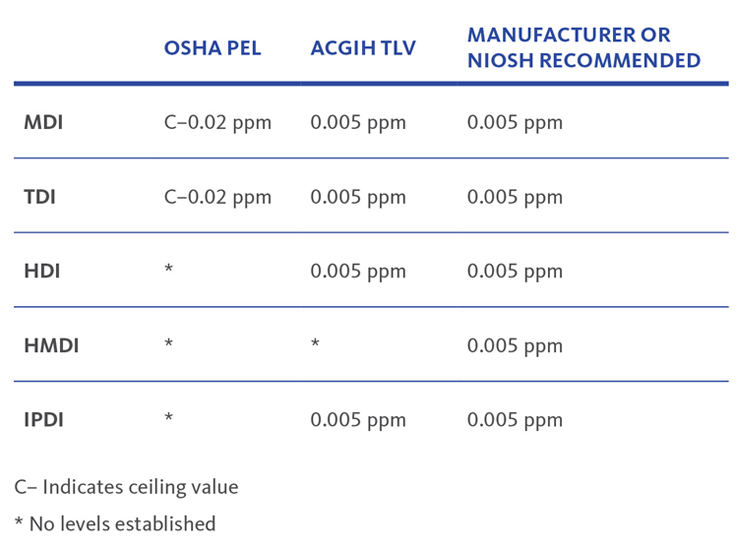
Exposure limits
Since diisocyanates are highly toxic, you should understand the exposure limits. In order to keep workers safe, follow the guidelines from OSHA and the American Conference of Governmental Industrial Hygienists. They’ve established these permissible exposure limits and threshold limit values for several diisocyanates:
Related resources
Silent danger in the workplace
It’s colorless, odorless, and a danger for some workers. But there are things you can do to help keep carbon monoxide from becoming deadly. We have some tips.
Managing cell phone distractions
Cell phone use while driving is a distraction for your driver. We’ll explain how you help can manage it.






